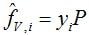In the Ideal Thermodynamic example, the vapor phase is governed by the ideal gas law (PV = RT). The enthalpy reference state is in the (ideal) vapor phase at Tref = 298.15 K. Reference pressure is required for entropy, and is Pref = 101325 Pa. Volume and density of the vapor phase follow from the ideal gas law. Enthalpy of the vapor phase follows from
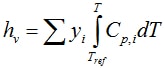
Entropy of the vapor phase follows from
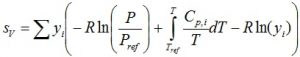
Fugacity coefficient of all components is unity, corresponding to an ideal gas. Fugacity for the vapor phase therefore is: