In the Ideal Thermodynamic example, the liquid phase is ideal, with activity coefficients of unity. The system is intended to be used for T < Tcrit,i for all compounds, so that all vapor pressures are finite and positive. Calculations that violate this constraint result an error.
Poynting correction and saturated fugacity coefficients are ignored, so that the liquid phase fugacity is given by
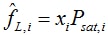
where the saturated vapor pressure only depends on temperature. The liquid phase enthalpy follows from:
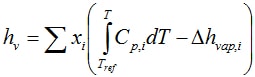
The liquid phase entropy follows from:
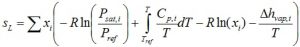
The liquid phase volume is ideal:
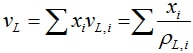
and the liquid phase density follows from ρL = 1/vL.
